Accelerating Drug Development Through Simulation and Optimization
Streamlining the Research Process
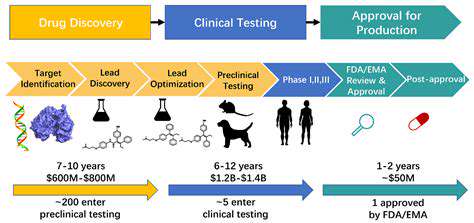
The acceleration of drug development relies heavily on refining the research process from early discovery phases through clinical trials. Optimizing lab workflows, adopting cutting-edge analytical methods, and implementing rigorous data governance frameworks are critical steps. Automating mundane tasks and eliminating procedural roadblocks allows scientists to focus on creative experimentation and in-depth analysis, paving the way for groundbreaking discoveries. Enhanced partnerships between academic institutions and pharmaceutical firms play an equally vital role in this ecosystem.
Effective data stewardship systems prove indispensable for monitoring research trajectories, maintaining data accuracy, and enabling swift information retrieval. Such systems empower investigators to spot potential issues at earlier stages and refine their approaches accordingly, conserving precious time and funding. Standardized experimental protocols and best practice guidelines substantially decrease procedural inconsistencies and errors, yielding more dependable outcomes.
Harnessing Cutting-Edge Technologies
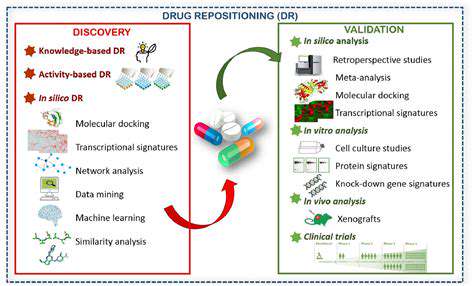
Emerging technological innovations like artificial intelligence (AI) and machine learning (ML) are transforming pharmaceutical research and development landscapes. These tools can sift through enormous datasets to pinpoint prospective therapeutic compounds, forecast effectiveness and safety parameters, and expedite clinical study designs. AI-powered analytical platforms operate at unparalleled speeds, uncovering hidden patterns and insights that would remain obscured through manual analysis alone. This accelerated processing capability dramatically shortens the timeline for identifying viable drug candidates.
High-capacity screening systems and sophisticated imaging modalities further propel the development timeline. Such technologies permit simultaneous evaluation of numerous chemical compounds or biological specimens, rapidly flagging potential therapeutic options. Advanced visualization techniques offer unprecedented glimpses into biological mechanisms, facilitating the creation of precision-targeted treatments.
Refining Clinical Trial Methodologies
Enhancing clinical study frameworks represents a pivotal factor in speeding pharmaceutical development. Adaptive trial architectures, which permit mid-study adjustments based on emerging data, enable quicker recognition of effective treatment modalities. This flexible approach can substantially decrease the duration and expense typically associated with clinical investigations. Concentrating on patient demographics most likely to respond positively to specific therapies ensures trial efficiency and clinically relevant outcomes.
Implementing novel participant recruitment tactics and incorporating digital health solutions can dramatically improve enrollment rates. These methods broaden patient access to clinical studies, ultimately shrinking the timeframe required to assemble complete participant groups and achieve statistical validity. Strategic recruitment approaches form the backbone of successful trials, where appropriate technological integration can markedly enhance both enrollment velocity and data quality.
Fostering Collaborative Synergies
Productive interdisciplinary cooperation among researchers, pharmaceutical corporations, regulatory bodies, and healthcare practitioners remains fundamental for expedited drug development. Transparent communication networks and structured data-sharing arrangements promote knowledge dissemination and hasten the transition from laboratory findings to clinical applications. Joint initiatives cultivate an environment conducive to innovation, significantly shortening the journey from concept to patient delivery. This collective mindset and cooperative methodology can radically compress the timeline for delivering novel treatments to those in need.
Developing comprehensive frameworks for ethical data exchange and knowledge transfer proves essential for maximizing efficiency while maintaining rigorous standards. Such structured approaches facilitate the circulation of ideas, experiences, and proven methodologies across diverse research teams and organizations, thereby amplifying the collective impact of pharmaceutical development efforts.